Methane Hydrate: Potential Fuel?
What is Combustible Ice?
This “combustible ice” was later revealed to be methane hydrate (MH), a mixture of methane (CH4) & water. Frozen water molecules form a crystalline lattice structure, trapping high concentrations of CH4 molecules.
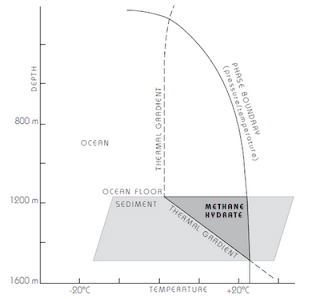
MH forms in 2 types of geologic environments with low temperatures & high pressure, where sufficient gas is present:
1. Below permafrost layers of rock
2. Beneath ocean floor (water depth>500m)
The MH deposits may be several hundred meters thick.
MH forms via the following methods:
1. Biogenic: biological activity in sediments
2. Thermogenic: geological processes deeper within the earth
Under atmospheric conditions, CH4 begins to escape the structure, causing the fizzling phenomenon. Put a lit match to the fizzling ice & it will burn. 1m3 of MH releases 164m3 of natural gas. MH was discovered in the 1970s during explorations by oil & gas companies, & was regarded as a peculiarity. Today, the industry frequently encounters MH as they venture farther out to sea in search of fossil fuels.
MH has been discovered in the following areas:
1. Permafrost of Mallik field, Beaufort-Mackenzie region above the Arctic circle, Canada
2. Nankai trough, offshore Japan
3. Gulf of Mexico
4. Prudhoe Bay & Kuparuk River oil fields, North Slope of Alaska
5. Carolina trough, offshore South Carolina, US
6. Messoyakha field, Western Siberia, Russia
7. Cascadia margin, off the coast of northern Washington & southern British Columbia, Canada
Many countries have begun to see the potential of MH to secure their energy resources:
1. Since 2000 – Japan has drilled 36 exploratory wells in Nankai trough – received largest investment & advanced field research
2. 2003 – an international consortium produced natural gas from MH in Canada – included Canada, Germany, India, Japan & US
3. 2007 – Japan & Canada began long-term production tests in Mackenzie delta
4. 2008 – US Dept of Energy (DOE) signed agreements for joint research on MH with India, Japan & Korea
5. 2009 – BPAX & US DOE explored sites for long-term production
Promise & Peril
CH4 is the primary constituent of natural gas, making it a valuable source of energy. Natural gas is a desirable fossil fuel because it is reliable, efficient & carbon-intensive. Estimated world MH reserves stand at 400 million trillion ft3, dwarfing the 5500 trillion ft3 of proven natural gas resources. This makes MH a promising candidate to be the fuel of the future. However the mining of MH is still in the R&D stage, although Japan is poised to be the 1st commercial producer of MH.
The main concern with MH mining is the instability of MH. MH are only quasi-stable; this property causes MH to dissociate when surrounding conditions pass out of the stability regime (when temperature increases or when pressure decreases). This poses a hazard on various levels:
1. Safety hazards during mining: uncontrolled gas releases during drilling; collapse of well casings; gas leakages to the surface.
2. Conversion of MH-bearing sediment into a gassy, water-rich fluid: may cause landslides, tsunamis – evidence of MH causing seafloor landslides has been found along the Atlantic Ocean margin in the US.
3. Acceleration of global warming: the content of CH4 in hydrates is approximately 3000 times more than that present in the atmosphere. CH4 is 10 times more effective than CO2 in causing global warming. Unsafe mining practices may yield rogue CH4 to the environment & increase the rate of global warming.
However with governments providing financial support for R&D in MH, some promising avenues have been identified:
1. Using CO2 injection: CO2 hydrates are more stable than MH. The trapped CH4 in the MH is exchanged with injected CO2, hence releasing CH4. This method is an attractive CO2 sequestration step as climate change legislation continuously dictates the reduction of CO2 emissions.
2. Extra-terrestrial MH: NASA has identified the existence of MH in ices on the moons of Saturn.
3. Synthetic MH: various efforts have been made to artificially create MH in the lab, such as ice-seeding method (Durham et. al, 2010). Research is underway to understand the geophysical properties of MH & production of pure MH.
Conclusions
As the world’s oil & gas reserves deplete amidst increasing demand for fossil fuel, MH is emerging to answer the call for alternative energy sources. Despite its immense potential, lack of knowledge on this substance has limited our strategies to make it a commercially viable energy source. More research & initiative is needed before we can tap MH as the substitute of oil & gas. Until then, this fiery ice will remain a fascinating treasure under the cold ice & deep blue.
References
1. Cohen, D.M. (2009, May). Hydrate production & CO2 injection: Two birds with one stone. World Oil Magazine, 230 (5), 21.
2. Dillon, W. (1992). Gas (Methane) Hydrates: A New Frontier. Retrieved from US Geological Survey site at http://marine.usgs.gov/fact-sheets/gas-hydrates/title.html
3. Durham, W.B., Kirby, S.H., & Stern, L.A. (1998). Polycrystalline Methane Hydrate: Synthesis from Superheated Ice, and Low-Temperature Mechanical Properties. Energy & Fuels, 12(2), 201-211.
4. Schmidt, G. (2004). Methane: A Scientific Journey from Obscurity to Climate Super-Stardom. Retrieved from NASA GISS Research Features at http://www.giss.nasa.gov/research/features/200409_methane/
5. Maize, K. (2008). Methane Hydrates: Gold’s Predictions Vindicated. Retrieved from Power Magazine Blog at http://www.powermag.com/blog/index.php/2008/11/13/methane-hydrates-golds-predictions-vindicated/
6. Mielke, J.E. (2000). Methane Hydrates: Energy Prospect or Natural Hazard? Retrieved from National Library for the Environment site at http://ncseonline.org/NLE/CRSreports/energy/eng-46.cfm?&CFID=1255395&CFTOKEN=96369856
7. Pentland, W. (2008, October). Methane Hydrates: Energy’s most dangerous game. Retrieved from Forbes.com site at http://www.cbc.ca/technology/story/2008/10/07/f-forbes-naturalgas.html
8. Walter, K. (1999). Methane Hydrate: A Surprising Compound. Retrieved from LNLL Science & Technology Review at https://www.llnl.gov/str/Durham.html







Comments